Translation Mechanisms
Archaeal ribosomes and evolution
Studies of fundamental processes, such as protein biosynthesis, provide a better understanding of evolution. Thus, the analysis of ribosomal RNA sequences of the small ribosomal subunit has led to a tree of life organized in three domains, bacteria, eukaryotes and archaea. Within this tree, archaea are close to eukaryotes and appear as a central piece to understand the evolution of living organisms. Efforts to better understand the diversity of archaea and their molecular mechanisms will provide important information
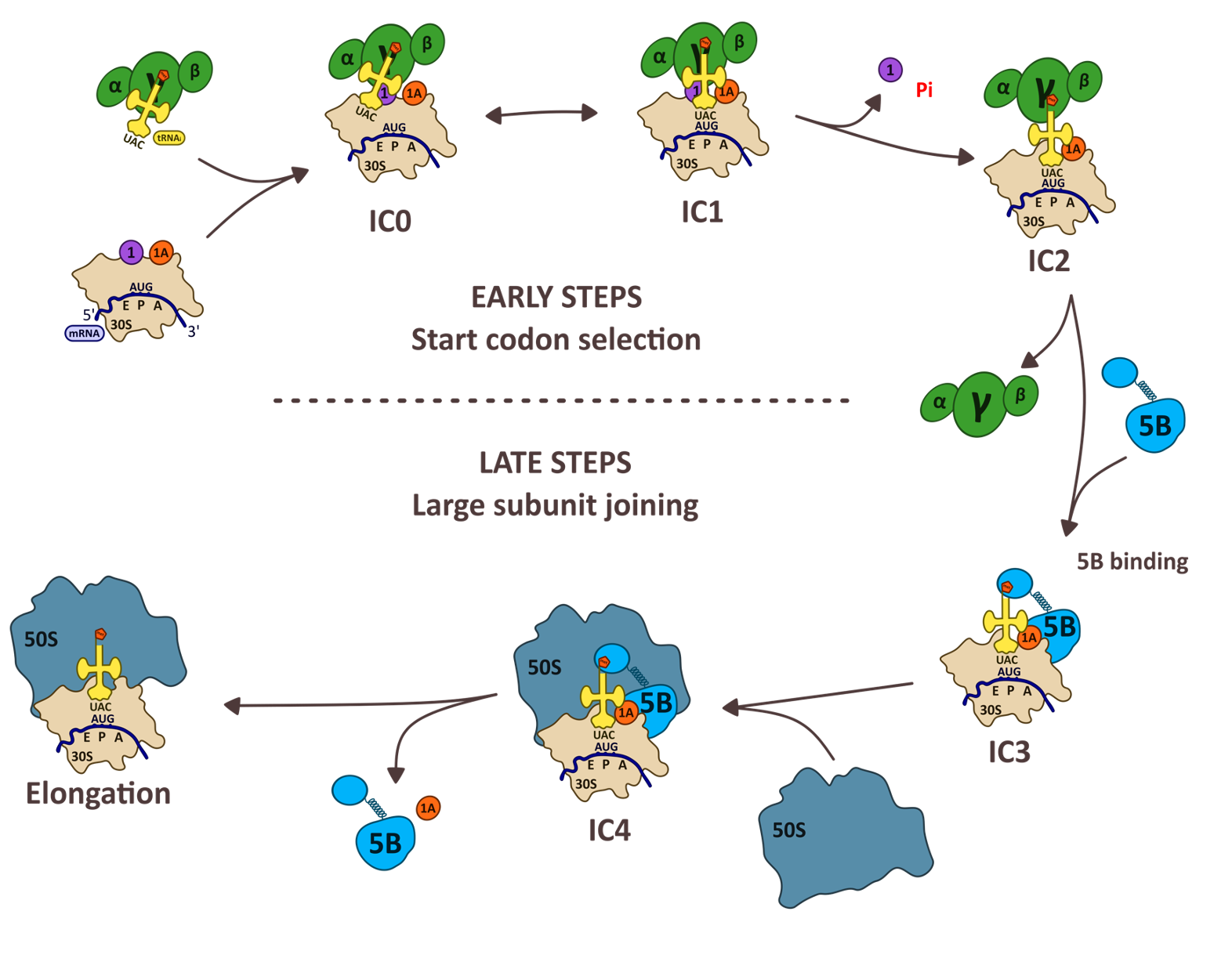
In archaea, mRNAs are not matured after their transcription, as in bacteria. Depending on the archaeal phylum, either mRNAs carrying Shine-Dalgarno sequences or mRNAs having very short 5' untranslated regions (leaderless) are preferentially used. However, other features of the translation apparatus bring archaea closer to eukaryotes. In particular, the archaeal ribosome and translation factors are of the eukaryotic type. Our team is mainly interested in ribosomal complexes of translation initiation. We study archaeal organisms and are also interested in bacteria and eukaryotes to better understand how the translation initiation mechanisms have evolved. We use X-ray crystallography, SAXS and cryo-EM to perform structural studies and we also developed biochemical strategies for functional studies.
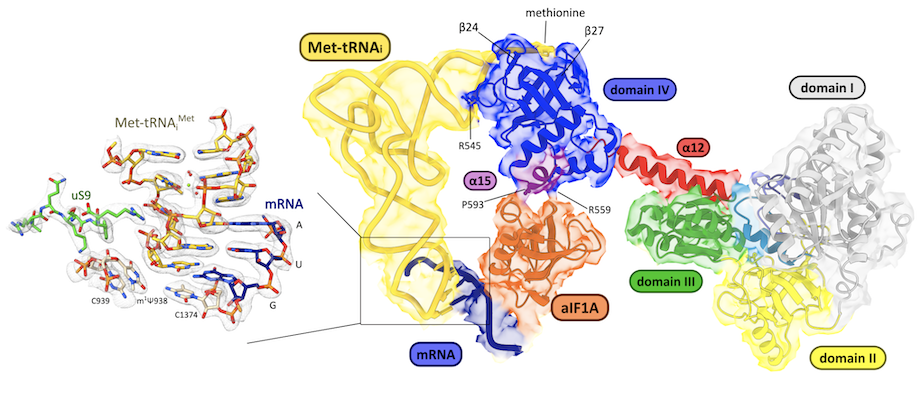
The ribosome of archaea is of the eukaryotic type. Archaea belonging to the TACK and Asgard branches, the closest relatives of eukaryotes, have ribosomes containing eukaryotic ribosomal proteins that are not found in the other archaea, eS25, eS26 and eS30. We recently studied the case of Saccharolobus solfataricus, a TACK archaea, which also has the particularity of possessing a majority (72%) of leaderless mRNAs. We determined cryo-EM structures of the small ribosomal subunit of S. solfataricus linked either to mRNAs with SD sequences or to leaderless messenger RNAs.
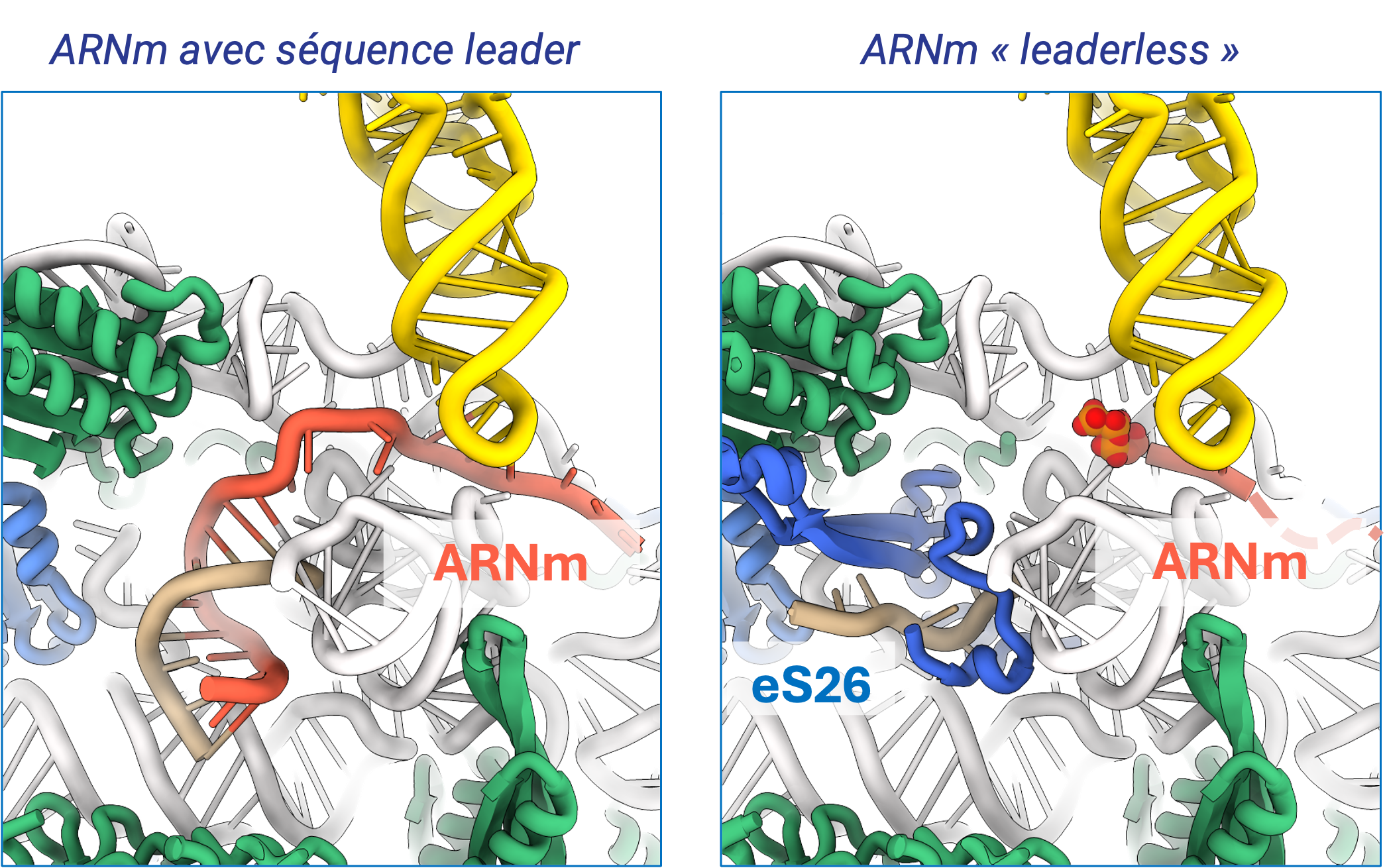
The cryo-EM structures show that eS25, eS26 and eS30 are bound to the small subunit as they are in eukaryotes. We identify two ribosomal proteins, aS33 and aS34, and an additional domain of the eS6 protein. Leaderless mRNAs are bound to the small subunit with the contribution of their 5'-triphosphate group. The archaeal eS26 domain binds to the mRNA exit channel wrapped around the 3' end of the rRNA, as observed in eukaryotes. Its position is not compatible with that of the SD:antiSD duplex. Our results suggest a positive role for eS26 in leaderless mRNA translation and possible evolutionary pathways from Archean to eukaryotic translation (Bourgeois G, et. al, Nat commun 2025).
The team is also studying other archaeal, bacterial and eukaryotic cell models in order to uncover the specific features of translation mechanisms.
Protein engineering
Site-specific incorporation of a β amino acid in a polypeptide would introduce flexibility in the main chain, thereby enlarging the geometric possibilities for protein folding. It has recently been demonstrated, using an in vitro cell-free translation system, that the ribosome is able to efficiently incorporate a β amino acid in a polypeptide. To enable in vivo synthesis of proteins containing β residues it is still necessary to design aminoacyl-tRNA synthetases capable of efficiently esterifying β amino acids on a specific tRNA. We work on this problem in collaboration with Philippe Marlière (Heurisko INC, USA) and Valérie Pezo (ISSB, Evry) using E. coli methionyl-tRNA synthetase (MetRS). Biochemical and structural studies of β methionine binding to MetRS give interesting clues for improving the catalytic activity of the enzyme towards β amino acids. In particular, we solved a 1.4 Å resolution X-ray structure of a MetRS:β-Met complex.Other protein engineering projects are underway.
eIF2 in cardiac diseases
Endoplasmic reticulum (ER) stress has emerged as an important mechanism involved in the pathogenesis of cardiovascular diseases. eIF2 is a factor involved in translation initiation, however it is also a key effector of the ER stress response. Disruption of ER function leads to protein accumulation, which triggers the Unfolded Protein Response (UPR). During this response, eIF2 is phosphorylated in its alpha subunit by the PERK kinase. This triggers a series of transduction events to reduce protein accumulation and ER stress. It is considered that a mild-to-moderate induction of this pathway favours a restorative autophagy-based response whereas severe or chronic ER stress response promotes apoptosis which contributes to the development of cardiac disease.
However, a recent discovery by our collaborator, C. Lemaire, showed that this pathway is controlled not only by eIF2 phosphorylation, but also by its acetylation. Moreover, deacetylation of eIF2 by the protein sirtuin 1 (SIRT1), protects cardiomyocytes from ER-stress induced apoptosis, by specifically attenuating activation of the eIF2 phosphorylation pathway and promoting autophagy.
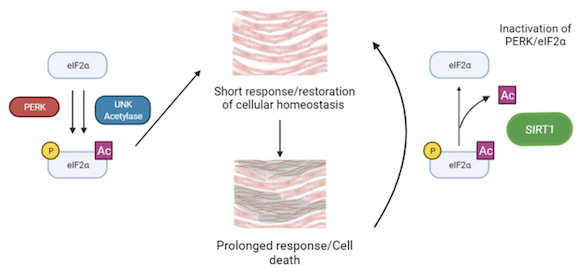
Interestingly, phosphorylation and acetylation levels of eIF2 seem to be related as overall increase of eIF2 acetylation is usually paired with an increase of phosphorylated eIF2. This SIRT1-eIF2 pathway has a potential to be used in drug therapy, as SIRT1 activity rates can be modified by Sirtuin Activating Compounds (STACs). Our aim is to understand the molecular basis of the interaction of SIRT1 with eIF2α, as well as the interplay between eIF2α acetylation and phosphorylation.
Main collaborations :
Laurence Blanchard and Arjan de Groot, BIAM CEA Cadarache
Franck Martin, UPR 9004, IBMC Strasbourg
Béatrice Clouet D’Orval, CBI, Toulouse
Didier Flament, IFREMER Brest
SOLEIL Synchrotron
Anh Tuân Phan (NTU, Singapore)
Philippe Marlière (Exapto, Palaiseau), Valérie Pezo (ISSB, Evry)
Christophe Lemaire, INSERM UMR-S1180
Selected publications :
- Paupelin-Vaucelle H, Boschiero C, Lazennec-Schurdevin C, Schmitt E, Mechulam Y, Marlière P, Pezo V. Cys-tRNAj as a Second Translation Initiator for Priming Proteins with Cysteine in Bacteria. ACS Omega. 2025 J10(5):4548-4560. doi.org/10.1021/acsomega.4c08326. eCollection 2025
Bourgeois G, Coureux PD, Lazennec-Schurdevin C, Madru C, Gaillard T, Duchateau M, Chamot-Rooke J, Bourcier S, Mechulam Y, Schmitt E. Structures of Saccharolobus solfataricus initiation complexes with leaderless mRNAs highlight archaeal features and eukaryotic proximity. Nat Commun 2025 16: 348 doi.org/10.1038/s41467-024-55718-5
Fedry J, Silva J, Vanevic M, Fronik S, Mechulam Y, Schmitt E, des Georges A, Faller WJ, Förster F. Visualization of translation reorganization upon persistent ribosome collision stress in mammalian cells. Mol Cell. 2024 Mar 21;84(6):1078-1089.e4, doi.org/10.1016/j.molcel.2024.01.01
Opuu V, Nigro G, Lazennec-Schurdevin C, Mechulam Y, Schmitt E, Simonson T. Redesigning methionyl-tRNA synthetase for β-methionine activity with adaptive landscape flattening and experiments.Protein Sci. 2023 Sep;32(9):e4738, doi.org/10.1002/pro.4738
Cardenal Peralta C, Vandroux P, Neumann-Arnold L, Panvert M, Fagart J, Seufert W, Mechulam Y, Schmitt E. Binding of human Cdc123 to eIF2γ. J Struct Biol. 2023 Sep;215(3):108006, doi.org/10.1016/j.jsb.2023.108006.
Kazan R, Bourgeois G, Lazennec-Schurdevin C, Larquet E, Mechulam Y, Coureux PD, Schmitt E. Role of aIF5B in archaeal translation initiation. Nucleic Acids Res. 2022, 50, 6532–6548, doi.org/10.1093/nar/gkac490.
Nigro G, Bourcier S, Lazennec-Schurdevin C, Schmitt E, Marlière P, Mechulam Y. Use of β3-methionine as an amino acid substrate of Escherichia coli methionyl-tRNA synthetase. J Struct Biol. 2020, 209, 107435, doi.org/10.1016/j.jsb.2019.107435.
Coureux PD, Lazennec-Schurdevin C, Bourcier S, Mechulam Y, Schmitt E. Cryo-EM study of an archaeal 30S initiation complex gives insights into evolution of translation initiation. Commun Biol. 2020, 3, 58, doi.org/10.1038/s42003-020-0780-0.
Schmitt, E, Coureux, P-D, Kazan, R., Bourgeois, G., Lazennec-Schurdevin, C., Mechulam, Y. Recent advances in archaeal translation initiation. Frontiers in Microbiology, section Biology of Archaea. Front Microbiol. 2020 Sep 18;11:584152, doi.org/10.3389/fmicb.2020.584152.




