Exosome secretion: beyond simple membrane fusion
In an article published in the Journal of Extracellular Vesicles, scientists show that the release of exosomes—extracellular vesicles that enable the removal of cellular waste and communication between cells—is more complex than previously thought. They demonstrate the involvement of two enzymes that cleave proteins involved in anchoring exosomes to the cell membrane. Because the activity of these enzymes is finely modulated, this discovery opens new perspectives for understanding how exosome release plays a role in normal and pathological cell function.
Extracellular vesicles: functions and release mechanisms
Extracellular vesicles (EVs) are membrane nanoparticles released by most cells. They contribute to the constancy of the internal environment by evacuating metabolic waste and acting as communication vesicles between cells. This homeostatic role presupposes the existence of precise mechanisms controlling their release, but these mechanisms remain poorly understood.
Exosomes are a subtype of EVs released by cells and produced from endosomes. Invagination of the endosome membrane leads to the accumulation of small intraluminal vesicles forming multivesicular bodies (MVBs). When MVBs are directed towards the cell membrane, it was thought that fusion of this MVB with the plasma membrane was sufficient to release exosomes into the extracellular environment. In an article published in the Journal of Extracellular Vesicles, scientists demonstrate that this last step is not sufficient to release exosomes. Using different murine and human cell lines of epithelial, neuronal or immune nature, they highlight an additional mechanism in the release of these vesicles involving two enzymes of the ADAM (a disintegrin and metalloproteinase) family, ADAM10 and ADAM17. These enzymes are proteases, present at the plasma membrane, which ensure the detachment of intraluminal vesicles retained on the cell surface. They cleave several adhesion proteins, including CADM1 and E-cadherin, which anchor the vesicle to the plasma membrane, thus finalizing the release of exosomes.
Regulation of exosome secretion and pathological implications
The intensity of ADAM-dependent exosome secretion is regulated by two signaling pathways with opposing effects on the bioavailability and activity of ADAM10/17 at the plasma membrane. MAPK kinases ERK1/2 stimulate the release of these EVs, while PDK1 ( 3-Phosphoinositide-Dependent Kinase-1 ) inhibits it.
The balance between the ERK1/2 and PDK1 pathways therefore determines the basal level of exosome secretion by the cell.
It remains to be seen, in a physiological context, which signals and receptors can modulate these pathways to adjust EV release. Given that deregulation of ERK1/2, PDK1, and ADAM10/17 has been associated with various diseases such as cancer and amyloid neurodegenerative diseases, scientists suspect that abnormal EV release could contribute to the progression of these pathologies.
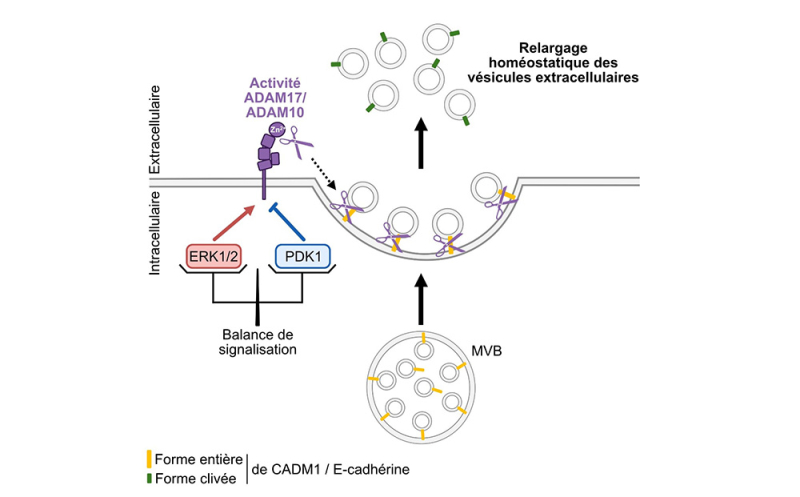
Figure: Involvement of metalloproteases ADAM10 and ADAM17 in the controlled release of extracellular vesicles. Under physiological conditions, the intensity of homeostatic release of EVs by ADAM10 and ADAM17 proteases present on the cell surface depends on a signaling balance that involves the kinases ERK1/2 and PDK1. ERK1/2 potentiates EV release by inducing ADAM phosphorylation, which increases their cleavage activity towards the adhesion proteins CADM1 and E-cadherin. Conversely, PDK1 opposes EV release by stimulating ADAM internalization, which reduces the membrane bioavailability of active ADAMs to ensure the detachment of EVs from the plasma membrane.
Publication : Bizingre C, Arellano-Anaya Z, Picard F, Pietri M, Baudry A, Roussel F, Bianchi C, Alleaume-Butaux A, Ardila-Osorio H, Romao M, Lavieu G, Raposo G, Schneider B. ADAM Sheddase Activity Promotes the Detachment of Small Extracellular Vesicles From the Plasma Membrane. J Extracell Vesicles. 2025 Jul;14(7):e70114. . PMID: 40673783; PMCID: PMC12269338.
Contact
Laboratory
Laboratory of Structural Biology of the Cell - BIOC (École polytechnique/CNRS)
École polytechnique - Building 84
Route de Saclay
91120 Palaiseau


